Marine snow and what we don't know
August 17th, 2025 | 10 min read

Last picture on land before setting sail upon the R/V Neil Armstrong from Woods Hole, MA on July 24, 2025.
Within a month of joining the lab, my professor threw me into the deep end — 4,472m deep to be exact. On July 25, 2025, I woke up to the ocean tumbling me out of bed. Whether I liked it or not, I was going to be in the North Atlantic Ocean for the next 16 days.
Learning the ropes
Walking onto the ship, I had no expectations in mind and every thing to learn. So many new acronyms and terms sloshed in one ear and came out the other. “We’ll deploy the CTD at 0300h. The Niskin bottle sign up sheet can be found here. We’re interested in learning more about PIC, POC, and DIC content at various depths around the pychnocline.” Geez, my advisor really did throw me into the deep end.

Over the course of 5 weeks, we deployed the CTD over 100 times with the help of the ship's crew. There are a total of 24 Niskin bottles on the CTD, along with various optical instruments strapped to the bottom of the chassis.
And I couldn’t help but to compare myself to the other scientists on board. They knew exactly how to navigate the ship, where everything was, what to do when samples come up from the ocean, and more. As for me, I couldn’t tell my left from my right, my starboard from my port — or is the other way around?

Scientists and ship crew would work together in shifts to deploy various instruments into the ocean like the shadowgraph imaging system depicted here.
I realized that the sooner I learn things, the sooner I could do useful things. And in order to learn quickly, I had to be comfortable asking tons of questions, even if it made me look super incompetent. But hey, what’s the worst they could ask me: “Is this your first time on a research vessel?” “Uhh — yeah.” When I owned up to my novice status, any feelings of being overwhelmed were replaced by a sense of confidence. I took the time to learn the basics, from tying knots to figuring out where the lab supplies were. The bowline knot and trucker’s hitch are my two favorites right now. By the third time I set up the CTD1 for deployment, I could do it with my eyes closed (though I probably shouldn’t given I’m on a rocky ship). And yes, I was getting my sea legs. Instead of popping 4 Dramamine tablets a day for motion sickness, I only needed one (or even none on days I forgot about it). I’ll acknowledge that I couldn’t have learned as quickly as I did if it weren’t for the other scientists’ patience and wisdom. Turns out there was no need to be intimidated by their experience.
Now that I had a grasp of how to operate on a ship, I could finally focus on doing experiments. What experiments though? I got so caught up on how to deploy deep sea instruments and tying knots I hadn’t paid too much attention to the science I was originally tasked to complete!
The research
The whole reason why my professor sent me out to sea (and has the funds to do so) is because our lab is interested in the convergent impact of marine viruses, minerals, and microscale physics on phytoplankton carbon sequestration. Yes, I know. Lots of terms. And what do they have to do with one another? Let’s start with carbon sequestration.
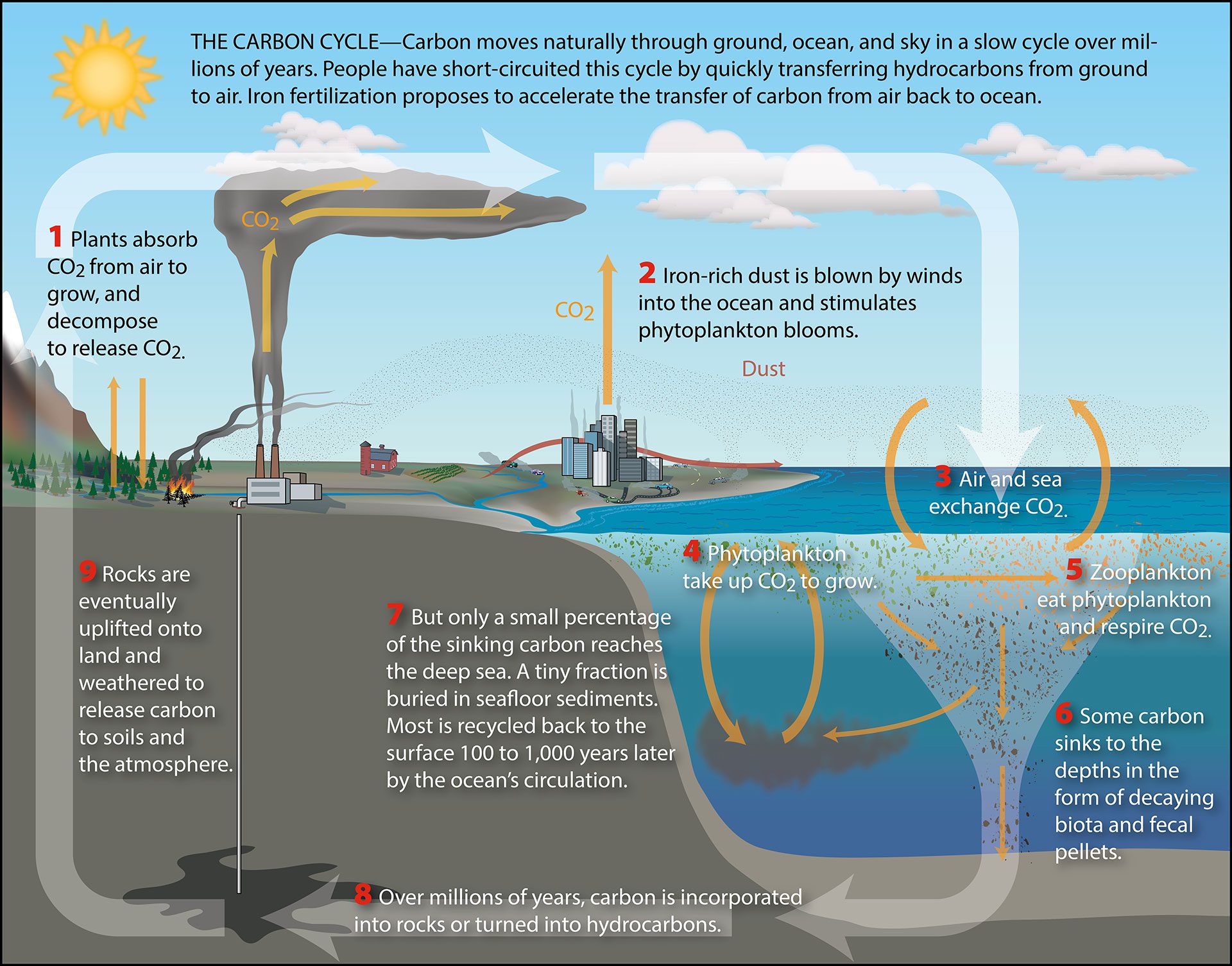
Diagram courtesy of Woods Hole Oceanographic Institution (WHOI).
Carbon sequestration refers to the exchange of carbon between the atmosphere and oceans. Carbon takes many forms, and the form it takes determines how it can move around. For instance, carbon dioxide gas in the atmosphere can be dissolved into the liquid body that is ocean.2 Once dissolved, it takes the form of carbonic acid, bicarbonate, and carbonate, each of which exists in chemical equilibrium with one another. But there’s more to the story beyond water and gas. In order to have the full picture of carbon fluxes within the ocean, we must also account for life. Marine microorganisms, like the various types of plankton, use this dissolved inorganic carbon and incorporate it into their biomass through none other than the process of photosynthesis. Great, now we have plankton growing off of dissolved carbon. Then what?

Prof. Kay Bidle walks through how the marine snow catcher system works.
The truth is, scientists don’t have a clear picture of what actually happens next. We have the general idea that the plankton will grow, multiply, and eventually die. Upon dying, the clump of biomass may aggregate with other clumps and sink to the bottom of the ocean.3 For something as grotesque as a hodgepodge of microscopic carcasses, fecal pellets, and goo, it has quite a pretty name: marine snow. This marine snow is the link that connects the flow of atmospheric carbon to the reservoir of carbon at the bottom of the ocean. But there are still so many open questions! What is the rate at which carbon is locked up into the ocean? What factors make this rate higher or lower? The answer to these questions have a direct impact on our climate models. If our current models overestimate the rate of carbon sequestration to the bottom of the ocean, this means, carbon dioxide gas is building up in the atmosphere faster than we thought. In other words — we’d be screwed! The scary part is that we have reason to believe that our ocean carbon sequestration models are grossly wrong. This is because we haven’t taken into account two sneaky little characters that potentially play a big role: marine viruses and bacteria.
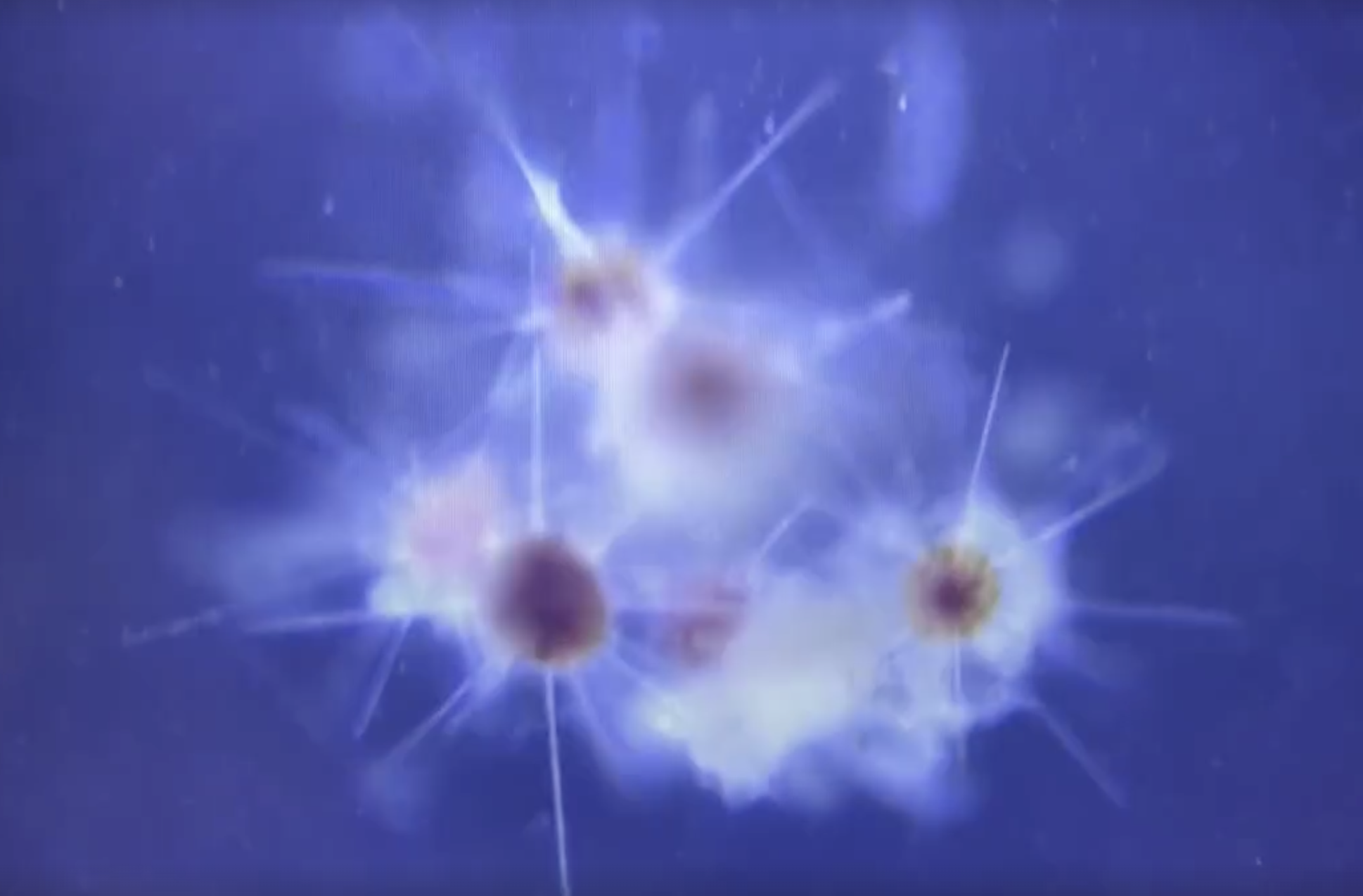
Acantharia are a type of plankton with radial symmetry. Six of them can be seen aggregated within this marine snow particle.
Yes, marine viruses and bacteria exist. Don’t worry, they only infect marine microorganisms like plankton 4. The thing is, these viruses and bacteria are found on marine snow particles chewing away at it.5 You may ask, “What’s wrong with bacteria eating marine snow?” This means that the carbon that was destined to be locked away at the bottom of the ocean doesn’t actually make it there. Instead, it’s eaten by bacteria, which recirculates the carbon to the surface of the ocean. If this surface carbon isn’t shunted away to the bottom, it reduces the ability of oceans to absorb more carbon dioxide from the atmosphere above it. More surface carbon means more atmospheric carbon dioxide, a greenhouse gas. And as you may be familiar with, more greenhouse gases throws off the hospitable balance our planet has maintained for much of human history. Although we’re studying a microscopic process, its planetary scale makes it a huge problem.
Through the lens of biophysics, we are asking questions that will directly inform our oceanic carbon sequestration model.
- At what rate does marine snow fall?
- How does marine snow form and break apart? Astronomers understand how stars are born and die. We should be able to understand the analogous processes for marine snow.
- How do microbial communities affect the composition and settling dynamics of marine snow.
- How are climate changes affecting the rate at which marine snow forms and falls?
Collaboration on the ocean
The best part of being on a research vessel is seeing the ways that other labs and scientist are tackling this problem. Members of the Van Mooy Lab are analyzing lipid6 content in plankton, the Bidle Lab study coccolithophores and their interactions with other marine microbes, and the Thamatrakoln Lab model how microorganisms respond to environmental changes. Also aboard the ship were members from the Rau Lab, UNH Phyto Lab, and Prakash Lab.

Scientist team photo on our last day in the North Atlantic Ocean before beginning our 50 hour transit back to Wood Hole, Massachusetts.
Because we were literally doing our work elbow-to-elbow, we can’t help but to collaborate. For instance, while I was waiting for my 96-well plate to be scanned by the microscope, I wandered over to the Bidle group to see what they were up to. Within half an hour, I ended up learning a lot more about coccolithophores, a type of plankton that makes these intricate shells made of calcium carbonate.7 It didn’t stop there though. When your research lab brews in the midst of all these other labs, you can’t help but to think about ways you can combine your efforts to tackle questions or carry out experiments that each lab couldn’t have done on their own. My lab focused on the sinking dynamics of marine snow. The Bidle Lab looks at coccolithophores. Together, we can ask: “what role do coccolithophores play in marine snow sinking dynamics?”

Simone Cuccagna operating a holographic camera to study settling dynamics of particulates like marine snow. This device is strapped to a gimbal to counteract the rocking of the ship.
And soon after posing questions, we could start answering it right away thanks to the tons instruments we had on board: confocal microscopes, cell sorters, a holography imaging system, and filtration systems. This isn’t even including the instruments that the research vessel came with, like a winch to deploy the CTD, marine snow catcher, and various nets to tow behind the boat. If we were doing this research apart back at our respective home campuses, it would have taken weeks just to set up video meetings and ship over samples to each other!
Conclusion and beyond
To make the most of research at sea, I learned how to be agile and efficient. The three most important things to do are to (1) collect samples/data, (2) generate/test new ideas, and (3) collaborate with other scientists. You’re best positioned to make the most of it while surrounded by a bunch of other great minds and a ship full of equipment.

Working from sun up to sun set.
This expedition at sea has been an amazing opportunity for me to grow as a scientist. Funds from organizations like the National Science Foundation (NSF) help make this possible, but sadly, these annual funds are being slashed by about 57% compared to the previous year. While it is all too easy to blame these changes on a single person or even a select few people, we scientists must also admit that our culture of secrecy and inaccessibility only exacerbates the disconnect with the public. These changes remind me that scientific communication does not (and should not) just come in the form of cryptic academic papers. It colors the way we talk about the world, see our own bodies/health, and imagine the technology that shapes our future.
-
I now know that “CTD” stands for “Conductivity, Temperature, Depth.” It’s basically an instrument with various sensors sent down the ocean with a bunch of open (Niskin) bottles. As we pull it back up, we remotely trigger the bottles to close, capturing the water at the current depth. ↩
-
Yes, gases can dissolve into liquids. In fact, every time you enjoy a fizzy drink, it is the dissolved carbon dioxide gas in the drink that tickles your taste buds. ↩
-
Fun fact: the material we know as limestone is actually the compaction of millions of years worth of phytoplankton bodies raised up to the surface of the Earth again. ↩
-
We think. ↩
-
Bochdansky, A. B. et al. Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. The ISME Journal 11, (2017). ↩
-
Lipids are a class of biochemical molecules that makes up fat and other cellular components. ↩
-
This is actually the same chemical compound as chalk! ↩
